Scientists have made a breakthrough in understanding the process that leads to a blood clot forming in the lungs – a condition that kills more than 2,000 people in the UK each year.
The clot forms a pulmonary embolism or blockage, cutting off blood flow to major blood vessels in the lungs.
In many cases, the blockage is caused by fragments that have broken away from a blood clot elsewhere in the body, such as a deep vein thrombosis in one of the legs.
The fragments are transported to the lungs via the bloodstream.
...we have identified new targets for drugs to stop fragments of a thrombosis breaking away and causing an embolism...
In a paper published in the scientific journal PNAS, researchers from the Universities of Leeds and Sheffield report on the role played by a protein called fibrin in stabilising the original clot, to prevent bits of clot from breaking loose.
Blood clots – why do they fragment?
A blood clot forms if a blood vessel is ruptured, and its function is to stem blood loss. But sometimes, a clot can form inside a blood vessel in the absence of any injury, and this causes a thrombosis.
The research team used animal studies involving mice to investigate a key chemical building block of the clotting protein fibrin, known as γ-chain cross links. The scientists found that the γ-chain cross links give the fibrin its stability through enhanced resistance to rupture and clot fragmentation.
In the study, they looked at clot behaviour in mice that were genetically modified so they could not produce the stabilising γ-chain cross links in the fibrin, and compared them with mice that could.
The results revealed that the clots without the γ-chain cross links were more unstable and more likely to fragment and produced more associated embolisms.
Fibrin – ‘not strong enough to hold clot in place’
Dr Cédric Duval, the study’s lead author and lecturer in the School of Medicine at Leeds, said: “What we believe is happening is that without the γ-chain cross links, the fibrin is not strong enough to hold the clot in place against the forces generated in the body from muscle movement and from blood flow.”
Professor Robert Ariëns, also from the School of Medicine at Leeds, who supervised the research, said: “The findings reveal the importance of the γ-chain cross links. These are the structural supports in the fibrin that keep the clot in place.
“By identifying the structural dynamics of this mechanism, we have identified new targets for drugs that could be developed to stop fragments of a thrombosis breaking away and causing an embolism in the lungs.
“This is a disease that is a major cause of disability and death around the world.”
The research findings are an acknowledgement of a long-held suspicion on the part of Professor Ariëns that the structure of fibrin plays a role in the fragmentation of clots – but until now, there was no scientific evidence.
He said: “I have always thought that the remarkable elasticity of fibrin, which has been described as like rubber or spider silk would be important to prevent clot fragmentation and thus thromboembolic disease.
“I was astounded to see the level of differences in pulmonary embolism that resulted from a genetic mutation that resulted in reduced elastic recovery of the fibres. So, when I saw the results, it definitely was a wow moment and I also had that ‘...I told you so feeling’.”
The research was funded by the British Heart Foundation.
Further information
For more information, contact David Lewis in the Press Office at the University of Leeds by email on d.lewis@leeds.ac.uk.
The paper – Elimination of fibrin γ-chain cross-linking by FXIIIa increases pulmonary embolism arising from murine inferior vena cava thrombi – is published in the Proceedings of the National Academy of Sciences in the US.
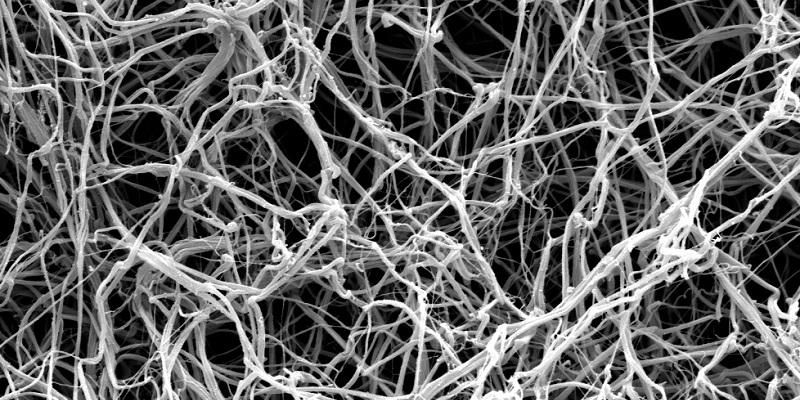
Top image shows fibrin fibres as seen by an electron micrscope at x 10,000 magnification. Credit: University of Leeds