A visualisation made from nearly 100,000 electron microscope images has revealed the ingenious way a protein involved in muscle activity shuts itself down to conserve energy.
The protein, called myosin, is known as a molecular motor because of the way it interacts with other proteins and energy molecules to generate force and movement. It is found inside muscle fibres where it forms long myosin filaments made up of hundreds of individual myosin molecules.
“Myosin is like a Brompton bicycle, kept in a folded state when not needed, and able to be quickly unfolded.”
When muscle activity ceases, the process of forming the myosin filaments goes into reverse: the filaments decouple and return to the individual myosin molecule state.
The visualisation – developed by scientists from Leeds and East Carolina University in the US – has revealed how the structure of the molecule changes. Their findings, the Structure of the shutdown state of myosin-2 have been published in the journal, Nature.
Structure of myosin
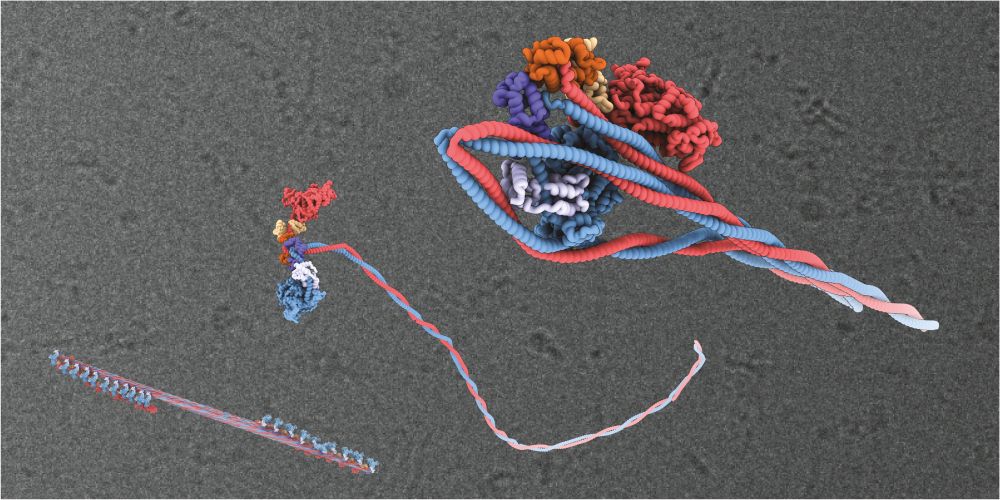
By combing 96,000 electron microscope images, the scientists were able to see how the myosin molecule adopts an inactive form in unprecedented detail. The tail of each molecule wraps itself around the head and is locked in place by key molecular interactions. That process shuts down its activity and makes it easier for the molecule to be recruited to where it is next needed.
Professor Michelle Peckham, from the Astbury Centre for Structural Molecular Biology at Leeds, supervised the research. She said: “The analogy here is that the folded myosin is like a Brompton bicycle, kept in a folded state when not needed, and able to be quickly unfolded when it is, by releasing a simple catch.
“The compact folded myosin is also more easily transported through a crowd to where it’s needed.”
Scientists have been aware of the role of myosin in muscle activity for decades. But until now, they were unclear about how this inactive state was formed or how its formation was so highly controlled.
Understanding disease
There are genetic mutations of myosin linked with certain diseases.
Professor Peckham explained: “Mutations in muscle myosin cause a wide range of muscle diseases. Our research into the structure of myosin and the way it functions help explain how mutations or defects in the protein may be causing disease. That opens the door to the possibility that scientists can develop therapeutic approaches to ensure myosin functions normally.”
The Astbury Centre for Structural Molecular Biology is an interdisciplinary research centre involving biologists, physicists and chemists to investigate the molecular basis of life. One of the major research themes is to understand the process of protein folding.
The research was funded by the Medical Research Council and Wellcome Trust.
Further information
Top image: The three states of myosin – at the top, in its inactive state; in the middle, its active state and at the bottom combining to form myosin filaments.
For further information, please contact David Lewis in the press office at the University of Leeds: d.lewis@leeds.ac.uk